
| Quality and Regulatory Services, Inc. provides FDA compliance solutions to medical device manufacturers. |
Quality and Regulatory Services, Inc. helps U.S. clients take medical devices to Europe and foreign clients bring medical devices to the U.S.A.
| Click on an item to learn more about our services | 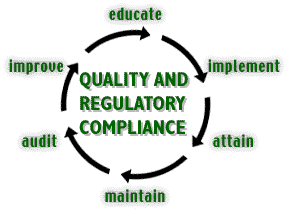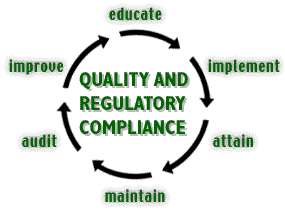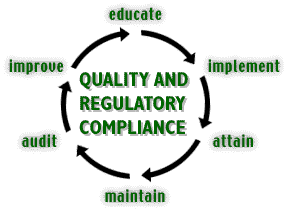 |
Quality and Regulatory Services, Inc. provides support, assistance, and training for all Center for Devices and Radiological Health/FDA compliance needs including:
| QSR/CGMP implementation and audits | |
| Product and process validation for hardware and software. | |
| Investigational Device Exemptions (IDEs) | |
| Premarket Approval (PMAs) | |
| Premarket Notifications (510(k)s) | |
| FDA 483 or Warning Letter recovery | |
| Radiological Health | |
| ISO 13485 implementation and audits | |
Home |
CEO Profile |
Philosophy |
Contact Us
|
Educate |
Implement |
Attain |
Maintain |
Audit |
Improve
| |
| CONTACT QUALITY AND REGULATORY SERVICES, INC. |
|
Tel: 925-787-2421 Email: |
© Copyright 1998-2011. All Rights Reserved.
Quality and Regulatory Services Inc.